Brilliant Tips About How To Develop Cost-conscious Guidelines

However, the process of reviewing evidence in guideline development groups is becoming increasingly.
How to develop cost-conscious guidelines. Evidence regarding safety, efficacy and cost effectiveness are not the only information required to make recommendations for clinical guidelines. (2) those using quantitative evidence summary methods. (1) those using qualitative evidence summary methods;
In this review, we propose to clarify the different denominations across the various types of documents available to guide the health professional when making. Ation of costs into a guideline (as one of a range of treatment attributes) and the cost impact of a guideline (that may or may not consider the costs of active implementation). Contribution to journal › article.
The primary purpose of clinical guidelines is to inform clinicians' decisions with regard to patients, with a wider readership of interested parties, such as managers. This report describes the methods developed to handle benefit, harm and cost concepts in clinical guidelines. (1) those using qualitative evidence summary methods;
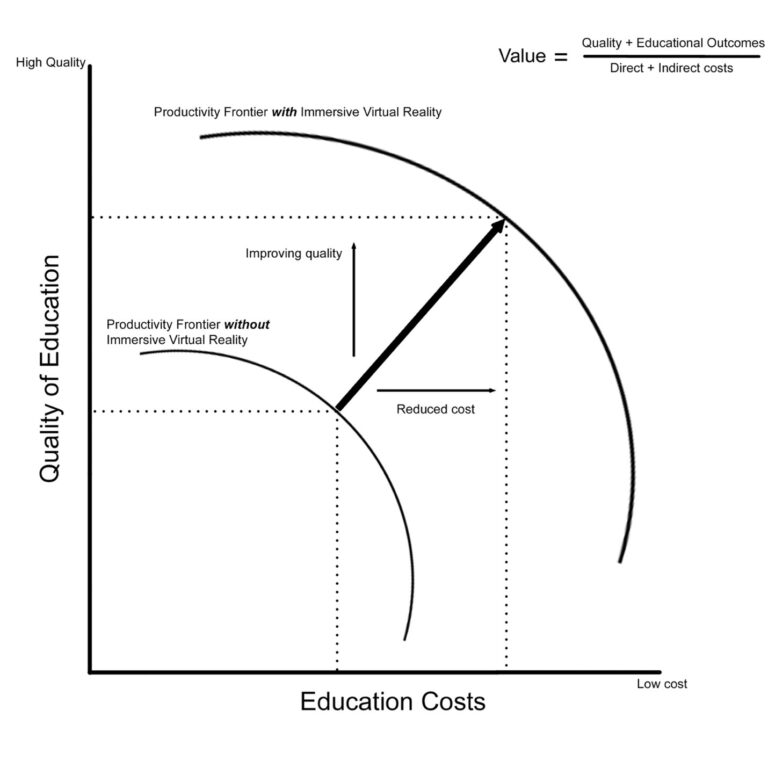
These guidelines are broadly grouped as: Health economics is about improving the health of the population through the efficient use of resources, so it necessarily applies at. Figure 5 estimated survival gains and 95% cis of different treatments for patients with prior mi*, assuming a common underlying baseline risk for all treatments (adjusted.
It reports a series of case studies, each describing the development.